|
Chemical Equations and Mole Relationships Dr. Walt Volland revised October 12, 2009 |
|
|
|
Example: |
|
Burning carbon and carbon containing compounds in air can produce carbon monoxide. This is one reason why carbon monoxide detectors are marketed for consumers. Space heaters have sometimes killed unsuspecting people. What they didn't know did hurt them. Carbon monoxide is poisonous. It is cumulative and even if it doesn't kill it can cause chronic illness and brain damage. |
|
2 C(s) + O2(g) -----> 2 CO(g)
|
|
This equation can be viewed in terms of the atomic and molecular level. Two atoms of carbon must react with one molecule of oxygen. Two molecules of carbon monoxide are produced. return to top of page |

|
|
|
|
|
|
|
|
The reaction between nitrogen and oxygen to produce nitrogen dioxide is analyzed here. The balanced equation is N2(g) + 2 O2(g)---> 2 NO2(g) |
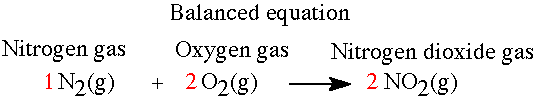
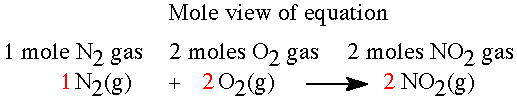

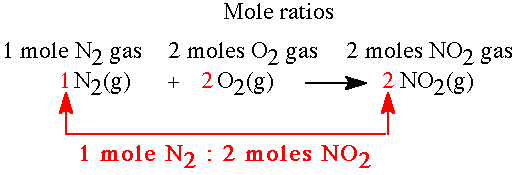
Exercise: What is the mol ratio for nitrogen to oxygen for the equation above?
Answer: 1 mole N2 : 2 moles O2
|
|
|
|