|
Chemistry ---------------------- |
|
Name______________ |
|
Dr. Walt Volland |
|
Short answer essay questions
return to homepage
Chemistry ----------------------
-----------------Quiz 8--------------------- Name______________
Dr. Walt Volland
50 point Short answer essay questions
|
1. 10 points What makes H2O and CH3CH2OH completely miscible? What makes any pair of materials "miscible"? Give answer in terms of molecular structures and interactions. |
2. 10 points How does the temperature of a
solvent like water influence the solubility of a dissolved
gas like CO2?
Give an example from your experience. 4 points The illustration below shows a gas
and a solution of the gas at equilibrium. What will happen
to the gas pressure in the gas state, if the volume of the
space is reduced from 100 mL to 67 mL? What happens to the
number of dissolved gas molecules at this new pressure? What
happens to the number of molecules in the gas state? 6
points
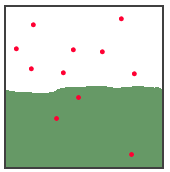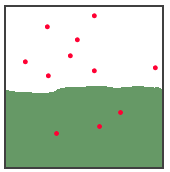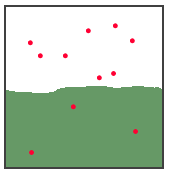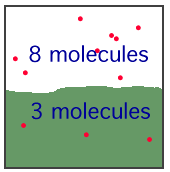
3. 10 points Write the formulas for the solute
ions in a 0.034 M Al(OH)3
aqueous solution. Give
the molarity for each ion. Show set ups for the
calculations.
Multiple choice question with short answer (2 points each, 20 points possible)
1. Which of the following is a
nonelectrolyte? Tell what an nonelectrolyte is. a. HCl(aq) b. NaOH c. H2O d. LiCl
2. What is the molarity of
the solution that results from mixing 3.0 mols of HBr(g) and
enough water to make 1200 mL of solution? Set ups are
shown Recall 1 mol HBr = 81 g
HBr a. 0.0025 b. (3.0) / (1.2) c. 3.0 ( 1.2)/ 81 d. (81)(3.0)/1.2 e. (3.0)/(81)(1.2)
3. How many mols of NaOH
are in 300 milliliters of 0.25 M NaOH ? What ions are in
solution? Set ups are shown 1 mol NaOH = 40 g NaOH a. 300 / .25 b. (300/1 ) (1/ 1000) ( 0.25
) c. 0.25 / .300 d. 40 ( 0.25 ) ( 1/ 0.300)
4. What is the molarity of a
mixture of 3.0 mols of KOH and enough water to make a
mixture with a volume of 0.800 liters? Set ups are shown.
Justify your answer. Use an explanation that refers to
units. 1 mol KOH = 56 g KOH a. 3.0 / 800 b. 3.0 / .800 c. 800 / 3.0 d. 56 (3.0 ) ( 0.800 )
5. What is the molarity when 240 ml
of 8.0 M HF solution is added to enough water to make a
total mixture of 1.20 liter ? Set ups are shown Tell what
makes your choice correct. a. 8.0 ( .240 / 1.20 ) b. 8.0 ( 1.20 / 0.240) c. 1.20 ( .240 / 8.0 ) d. 1.20 (240 / 8.0)
6. How many grams of
NH3
are in 300 ml of a 6.0 M NH3
solution? Set ups are shown 1 mol
NH3
= 17 g NH3 a. 17/ 0.300( 6 ) b. 17 ( .300 ) c. 6 ( 0.300) 17 d. 17 e. 17 ( 6 ) / .300
7. Which of the following is
expected to be soluble in H2O? Justify your
answer. a. CH4 b. CH3OCH3 c. SiO2 d. CH3SCH3 e. HCl
8. Henry's law can be used to
concentrations for solutions of water and which of the
following? Justify your answer with a brief explanation of
Henry's law. a. NaNO3(s)----------------- b. CH3CH2OH(l) c. NO2(g) d. HC2H3O2(l)
9. Solutions transmit light and
appear to be transparent. Justify your answer in terms of
particle sizes in solution. a. True----------------------------------- b. False
10. Which of the following will
produce a higher dissolved oxygen concentration? Justify
your answer. a. water and oxygen at a pressure of
0.05 atm at 20oC b. water and oxygen at a pressure of
0.05 atm at 40oC c. water and oxygen at a pressure of
0.15 atm at 10oC d. water and oxygen at a pressure of
0.15 atm at 40oC
return
to homepage