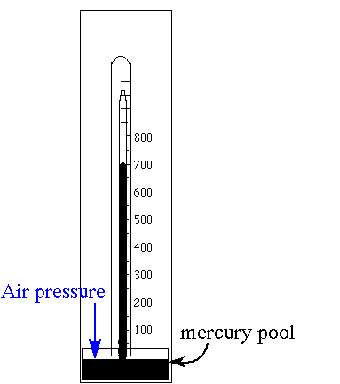
A barometer is shown in the illustration. What is the approximate indicated air pressure in mm Hg? What is this in atmospheres? Would this be classed as HIGH pressure? The atmosphere is typically 20% oxygen and 80% nitrogen. What is the partial pressure of oxygen at this pressure? What is the advantage of placing a carbon monoxide poisoning victim in a hyperbaric chamber ? Justify your answers.