Due
Monday 11:59 PM November 22, 1999
Short essay questions
Quiz 7: Solids,
Liquids & Gases: States of Matter
|
1. |
10 points |
|
|
----- |
----- |
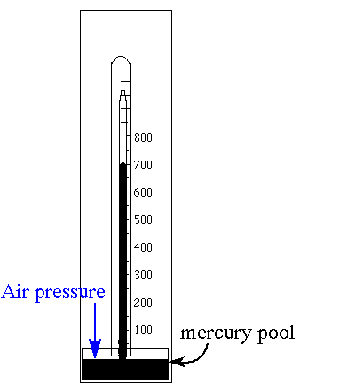
A barometer is shown in the illustration. What is the approximate indicated air pressure in mm Hg? What is this in atmospheres? Would this be classed as HIGH pressure? What would happen if to the mercury level if the density of the atmosphere above increased? Justify your answer.
|
|
2. |
----- |
10 points |
|
----- |
A balloon initially had a volume of 3000 liters at 20oCelsius and 1.0 atmospheres. What is the volume of the balloon at 10o Celsius and a pressure of 0.95 atm? Show set ups for calculations.
Describe how the gas volume would change when there is a decrease in temperature for the gas. |
|
3. |
----- |
8 points |
|
----- |
Explain what would happen to the boiling point and freezing point for water if there was no such thing as hydrogen bonding. Tell what other forces exist between water molecules besides hydrogen bonding. Justify your answer. |
|
4. |
----- |
8 points |
||||||||
|
----- |
Which of the four: diethyl ether, ethyl alcohol water or ethylene glycol, has the weakest intermolecular forces? Justify your answer. If possible tell what the approximate boiling point for each liquid would be if the opposing pressure was lowered to 400 torr (400 mm Hg)? Compound diethyl ether ethyl alcohol water Boiling point at 400 torr ------------------------------- ------------------------------- -------------------- |
|
5. |
9 points |
|||||||||||||
|
Which of the following gases has the smallest density at STP? How does density relate to molar mass? Calculate the densities to support your answer. Which would be the best to use to fill a lighter than air balloon? Explain your answer. Show a sample calculation. Compound N2 He CO2 Density at STP ----------------- ----------------- ----------------- Molar mass ----------------- ----------------- ----------------- |
|
True false 1 point seach |
||
|
1. |
A simple cubic unit cell has one "atom" in the cell. |
|
|
2. |
|
|
|
3. |
The body centered cubic unit cell packs atoms more efficiently than simple cubic. |
|
|
4. |
Molecules like H2 interact through dipole-dipole forces and hydrogen bonding. |
|
|
5. |
Hydrogen bonding can exist when molecules have any of the following bonding combinations. H-F, H-O, H-N. |