


|
Intermolecular
forces dipole-dipole, London forces, hydrogen bonding versus
Covalent bonds
|
Dr. Walt
Volland, all rights reserved 1998-2011 revised Nov 3, 2011 |
|
Attractions between molecules are
classified as |
- Dipole-dipole
interactions , example: ammonia, NH3
- London forces also known as van
der Waals forces, example: methane, CH4
- Hydrogen bonding example: water, H2O .
|
|
Relative magnitudes of
forces |
- The relative size of these
interactions is important so the relative effects are
understood.
- The relative typical strengths for the
different interactions are listed here in descending strength.
|
-
Covalent bonds
>
|
Hydrogen bonding
> |
Dipole-dipole interactions
> |
London
forces |
- 400 kcal
>
|
12-16 kcal > |
2-0.5 kcal > |
less than 1 kcal |
- From this we can see that normal covalent bonds
are almost 40 times the strength of hydrogen bonds.
-
- Covalent bonds are almost 200 times the strength of
dipole-dipole forces, and more than 400 times the size of
London forces.
|
- Dipole-dipole
interactions
|
- Dipole-dipole interactions
exist between molecules that are permanently polar. This requires the
presence of polar bonds and a asymmetric molecule. These
molecules have a permanent separation of positive and
negative charge. In the illustration the H end of HCl is
permanently slightly positive charge. The Cl end of HCl
has a permanent slight negative charge. the "H" in one
molecule is attracted to the "Cl" in a neighbor. The
intermolecular force is weak compared to a covalent bond.
But this dipole-dipole interaction is one of the stronger
intermolecular attractions.
|

|
London forces exist in nonpolar
molecules.
These forces result from temporary
charge imbalances. The temporary charges exist because the
electrons in a molecule or ion move randomly in the
structure. The nucleus of one atom attracts electrons form
the neighboring atom. At the same time, the electrons in one
particle repel the electrons in the neighbor and create a
short lived charge imbalance. |
 |
- These temporary charges in one
molecule or atom attract opposite charges in nearby
molecules or atoms. A local slight positive charge d+
(lower case Greek delta) in one molecule will be attracted to a temporary instantaneous slight d-
negative charge in a
neighboring molecule.
-
-
|
- London forces in
hydrocarbons and organic molecules
|
- The temporary separations of
charge produce London force attractions are what
associate one nonpolar organic molecule with its neighbors.
The possibilities for these interactions go up with
increasing molecular size and surface area. A larger surface
increases the chances for the "induced" charge
separations to interact. If the molecules are linear they have more
surface area than if they are folded into a sphere. The
linear molecules have higher melting and boiling points
because of the increased attractions.
|

|
Hydrogen bonding is a unique type
of intermolecular molecular attraction. There are two requirements.
First, there must be a covalent bond
between a H atom and either F, O, or N. (These are the three
most electronegative elements.)
- Second, the H atom in the polar bond -O-H, -N-H, F-H inteact with a lone pair of
electrons on a nearby atom of F, O, or N in another molecule or for BIG molecules in another part of the molecule.

|
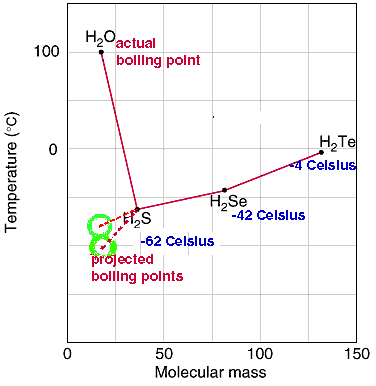
- The normal boiling point for
water is 100oC. The graph below shows how high this boiling point is compared to the predicted ( green circles) boiling points.
-
- The observed boiling point of 100oC is almost four times greater than the expected value at about -80oC or -100oC . The predicted boiling point (green circles) from the
trend of boiling points for
H2Te,
H2Se,
H2S
and H2O
is very low. If the trend continued the predicted boiling
point would be below -62 oC.
The "anomalous" boiling point for water is the result of
hydrogen bonding between water molecules.
|
- When can hydrogen bonding
exist?
|
|
Possible combinations where
hydrogen bond can exist.
The first entry shows the covalent
bond to the O or N atom. These atoms form two and three
covalent bonds. The single covalent bond between O,N,F is
shown and the dashed line shows the hydrogen bond. NOTICE
the H atom is attracted to a lone pair on the nearby N, O, F
atom.
A covalent bond between -O-H
---- :O-
A covalent bond between -N-H-----
:O-
A covalent bond between F-H ------
:O-
A covalent bond between -O-H ----
:N-
A covalent bond between -N-H----
:N-
A covalent bond between F-H -----
:N-
A covalent bond between -O-H -----
:F-
A covalent bond between -N-H ----
:F-
A covalent bond between
F-H ------ :F-
|
- Hydrogen bonding in an ice
crystal model
-
|
- Summary on
hydrogen bonding
|
|
Hydrogen bonding is responsible for
the expansion of water when it freezes. The water molecules
in the solid have tetrahedral spatial arrangement for the
two lone pairs and two single bonds radiating out from the
oxygen. The lone pairs on the "O" atoms are attracted to
nearby water molecules through hydrogen bonds. A cage like
structure results. The cage has an hexagon shaped
opening.

Click for more on hydrogen bonding in water |
- Exercise: Which of the
following molecules display hydrogen bonding?
|
|
Methane,
CH4 |
methyl ether,
CH3OCH3 |
|
Hydrogen peroxide,
H2O2 |
methyl alcohol,
CH3OH |
|
|
Answer: The hydrogen peroxide and methyl alcohol have hydrogen
bonding between molecules.
The methane lacks highly
electronegative atoms bonded to the hydrogen atoms. The
methyl ether has an oxygen but the carbons are bonded to the "O" . The hydrogen atoms are not bonded to very
electronegative "O" atom. |
|
Dr. Walt
Volland, all rights reserved 1998--2011
|
revised November 3, 2011





![]()


